Temperature-Dependent Degradation of Pesticides: Theory, D.L. Nofziger
General Description Model Description Simplifications Glossary Bibliography and Contributors
Temperature-Dependent Degradation
Degradation of pesticides in soils is the result of a combination of chemical and biological events (Bollag and Liu, 1990, Wu and Nofziger, 1999). A first-order degradation model is usually used to simulate the variation of residual mass of a chemical compound in a soil system after its application (Dykaar and Kitanidis, 1996; Walker, 1974). The first-order rate constant or half-life in the degradation model is dependent on soil temperature. This dependence can be approximated by the Arrhenius equation (Rocha and Walker, 1995; Walker, 1974; Walker and Barnes, 1981). Soil temperature under field conditions varies with time and depth. Here seasonal changes in soil temperature are predicted with a sinusoidal model (Carslaw and Jaeger, 1959; Fluker, 1958; Hillel, 1982; Kirkham and Powers, 1972; Penrod et al., 1960). This program calculates the concentration of chemical as a function of time for a user-defined degradation rate, temperature conditions, and initial concentration. Results are shown for the temperature dependent rate and optionally for two constant rate at user-defined temperatures. Graphs of concentration as a function of time for different input parameters can be used to gain insight into the influence of each parameter upon the degradation process and the importance of temperature upon the degradation rate.
The temperature-dependent degradation model is composed of three components: (1) first-order reaction kinetics for the degradation process, (2) the Arrhenius equation for half-life dependence on soil temperature, (3) the sinusoidal function for soil temperature variation with time and depth.
First-order reaction kinetics : First-order degradation kinetics may be expressed as (Dykaar and Kitanidis, 1996)
![]()
where C is the concentration of the compound of interest; k is the first-order rate constant; and t is time. In practice, the first-order rate constant often is replaced by a half-life, H where H = ln(2)/k or 0.693/k. Equation 1 then becomes (Rocha and Walker, 1995)
![]()
If the degradation rate and half-life is not a function of time, the solution to equation 2 can be written as
![]()
where C(t) is the concentration at time t and Co is the initial concentration. This solution can also be written as
![]()
Temperature dependence of half-life: The Arrhenius equation is used to represent half-life dependence on soil temperature (Rocha and Walker, 1995; Wu and Nofziger, 1999)
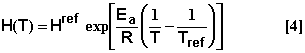
where Href is a reference half-life determined at the reference temperature of Tref, Ea is the activation energy of the reacting molecules; R is the universal gas constant; and T is the soil temperature in degrees Kelvin.
Sinusoidal temperature model: The annual variation of daily average soil temperature at different depths is described with the following sinusoidal function (Hillel, 1982):
![]()
where T(z,t) is the soil temperature at time t (d) and depth z (m), Ta is the average soil temperature (oC), A0 is the annual amplitude of the surface soil temperature (oC), d is the damping depth (m) of annual fluctuation and t0 is the time lag (days) from an arbitrary starting date (taken as January 1 in this software) to the occurrence of the minimum temperature in a year. The damping depth is given by d = (2D h/w )1/2, where Dh is the thermal diffusivity and w = 2 p /365 d-1 . More explanation of this equation and the parameters in it can be found in the documentation for the Soil Temperature Model.
This software calculates the concentration of chemical on a daily time step. Each day, equation 5 is used to estimate the temperature at the depth of interest. Then equation 4 is used to calculate the half-life for the current day. Finally equation 3b is then used to calculate the concentration on the current day from that on the previous day.
Assumptions and Simplifications
Documentation for the First-Order Degradation Model presents information on the assumptions inherent in it and conditions for which first-order kinetics are most likely to apply. This computer program implemented the first-order kinetics to simulate degradation without considering the effect of microbial growth on the degradation rate.
Similarly, the Soil Temperature Model presents simplifications made in that component of this model. Simplifications in that component include (1) the assumption that the temperature at the soil surface varies in a sinusoidal manner throughout the year; (2) at large depths in the soil, the soil temperature is constant and is equal to the average soil surface temperature; and (3) the thermal diffusivity is constant throughout the soil profile and throughout the year.
The Arrhenius equation applies only to moderate soil temperatures where soil enzymes are stable (Paul and Clark, 1989; Tabatabai, 1994). At high temperatures (usually between 60 and 70 oC) soil enzymes may be inactivated. The degradation rate may decrease with increase in soil temperature after certain critical point (generally 50 oC). This equation does not predict this decrease so it is appropriate only for temperatures below this critical temperature. So the model is appropriate only for soil moderate soil temperatures (generally 10 to 50 oC) where the enzymes are stable.
In addition to these general simplifications inherent in the equations, the implementation of the model makes incorporates the following additional simplifications.
1. The half-life calculated from equation 4 is applicable for the entire day.
2. The time lag t0 in equation 5 is taken as 10 days in the northern hemisphere and 192 days in the southern hemisphere.
3. The thermal diffusivity is taken as 7.0 x 10-7 m2 sec-1 (0.0604 m2 day-1).
Glossary
Activation Energy: Activation energy is the amount of energy that the reacting molecules must absorb from their surrounding environment in order to react.
First-order Rate Constant: First-order rate constant is a coefficient in an equation describing the rate of a first-order kinetics. The product of the coefficient and the reactant concentration yields the reaction rate.
Half-life: The half-life of a reaction is the time required for the concentration of one of the reactants to decrease to half of its initial value.
Microbial Degradation: Microbial degradation refers to the transformation of a chemical compound by the activity of microorganism, which is essentially intracellular, enzyme-catalyzed reactions encountered in the regular metabolic activity of microbes.
Reference Half-life: A reference half-life is a half-life of a reaction that is determined at a constant temperature. The constant temperature is called a reference temperature.
Bollag, J. -M. and S. -Y. Liu. 1990. Biological transformation processes of pesticides. P. 169-211. In H.H. Cheng (ed.) Pesticides in the soil environment: Processes, impacts, and modeling. SSSA Book Ser. 2. SSSA, Madison, WI.
Carslaw, H.S. and J.C. Jaeger. 1959. Conduction of heat in solids. Oxford University Press, New York.
Dykaar, B.B. and P.K. Kitanidis. 1996. Macrotransport of a biologically reacting solute through porous media. Water Resour. Res. 32:307-320.
Fluker, B. J. 1958. Soil temperatures. Soil Sci. 86:35-46.
Hillel D. 1982. Introduction to soil physics. Academic Press, Inc. 1250 Sixth Avenue, San Diego, CA 92101.
Kirkham, D. and W.L. Powers. 1972. Advanced Soil Physics. John Wiley & Sons, Inc., New York.
Paul, E. A., and F. E. Clark. 1989. Soil microbiology and biochemistry. Academic Press, Inc., 525 B Street, Suite 1900, San Diego, California 92101-4495.
Penrod, E. B., J. M. Elliott, and W. K. Brown. 1960. Soil temperature variation (1952-1956) at Lexington, Kentucky. Soil Science 88:275-283.
Rocha, F. and A. Walker. 1995. Simulation of the persistence of atrazine in soil at different sites in Portugal. Weed Research 35:179-186.
Tabatabai, M.A. 1994. Soil enzymes. p. 775-833. In Weaver et al. (ed.) Methods of soil analysis. Part 2. SSSA Book Ser. 5. SSSA, Madison, WI.
Walker, A. 1974. A simulation model for prediction of herbicide persistence. J. Environ. Qual. 3:396-401.
Walker, A. and A. Barnes. 1981. Simulation of herbicide persistence in soil; a Revised Computer Model. Pestic. Sci. 12:123-132.
Wu, J. and D. L. Nofziger 1999. Incorporating temperature effects on pesticide degradation into a management model. J. Environ. Qual. 28:92-100.
Contributors
This program was designed by Dr. D. L. Nofziger and Dr. J. Wu, Department of Plant and Soil Sciences, Oklahoma State University, Stillwater, OK 74078.
Send email to david.nofziger@okstate.edu
Last Modified: January 16, 2008.