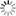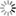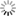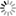Diffusion of solute in soil media, D.L. Nofziger
General Description Model Description Simplifications Glossary Bibliography and Contributors
Diffusion is the process by which matter is transported from one part of a system to another as a result of random molecular motion (Crank, 1957). Fick's law is used to simulate the rate of the diffusive mass transport (Crank, 1957; Kirkham and Powers, 1972). Combining Fick's law with the law of mass conservation produces a parabolic partial differential equation (PDE) for simulating diffusive mass transport under transient conditions (Allen III et al., 1988; Crank, 1957). Numerical and analytical solutions of the PDE have been developed for a collection of initial and boundary conditions. This program solves the equations for two sets of initial and boundary conditions. The first one demonstrates the one-dimensional spreading of a chemical plume of initial width h in a semi-infinite system with an initial concentration of zero. The second flow problem demonstrates diffusion into a soil containing no diffusing chemical at time zero. The effective diffusion coefficient in the soil is calculated taking into account the water content and porosity of the soil and the sorption of the chemical on the soil solids. Graphs of relative concentration as a function of distance for selected times or as a function of time for selected positions can be displayed.
Governing Partial Differential Equation: Solute diffusion in a soil medium with a constant water content and diffusion coefficient is often described using the equation (Carslaw and Jaeger, 1967; Crank, 1956;Kirkham and Powers, 1976)
![]()
![]()
where C = C(x,t) is the concentration of the solute in soil solution at position x and time t, q is the volumetric water content of the soil medium, and DE is the effective diffusion coefficient of the solute in a medium. The effective diffusion coefficient of a solute in a soil system can be estimated from the solute's diffusion coefficient in water, soil porosity, and soil water content using the so-called MQ model (Millington and Quirk, 1961)
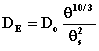
where Do is the solute diffusion coefficient in water and qs is the porosity of the soil.
Initial and Boundary Conditions: This program supports two sets of initial and boundary conditions.
Case 1: This case represents a scenario where the diffusing substance has an initial concentration Co in soil water from position zero to position h (called the mixing depth in this model). The diffusing substance has an initial concentration of zero at other positions. At times greater than zero, the material diffuses through the soil, but none of the material diffuses out of the soil at position zero. That is
![]()
and
![]()
The solution to the partial differential equation with this set of initial and boundary conditions is
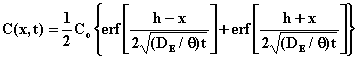
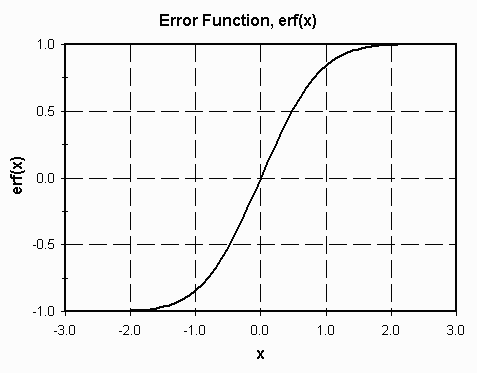
where erf(z) is the error function.
Case 2: The second set of initial and boundary conditions simulates the spreading of a diffusing substance into a soil where the concentration of the substance in soil water at the inlet is maintained at a constant, Co . Mathematically, these initial and boundary conditions can be expressed by
![]()
and
![]()
The solution to the partial differential equation with this set of initial and boundary conditions is
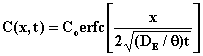
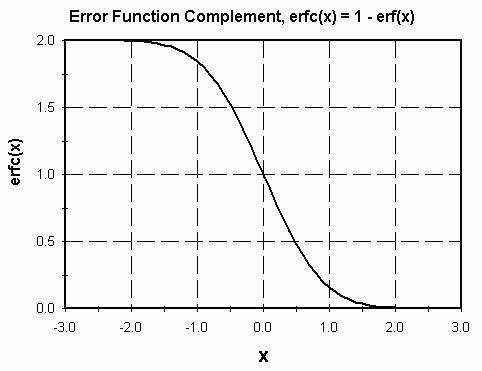
where erfc(z) = 1-erf(z) is the error-function complement.
The solutions described above are for chemicals that are not adsorbed on the soil surfaces. When sorption occurs, it reduces the effective diffusion coefficient. In that case, the effective diffusion coefficient DE can be estimated from
![]()
where R=1+rKd/q is the retardation factor of the chemical in the soil, r is the bulk density of the soil, and Kd is the partition coefficient of the chemical on the soil. The partition coefficient Kd can be approximated by the product of the organic carbon partition coefficient and the organic carbon content of the soil.
Assumptions and Simplifications
1. Diffusion coefficient may vary with the concentration of a diffusing substance and the medium in which a substance diffuses. In dilute solutions, such as soil-water solutions, the behavior of each diffusing molecule or ion is expected to be independent of the behavior of the others. The diffusion coefficient of a solute in a dilute solution can reasonably be taken as constant. In other systems, such as the inter-diffusion of metals or the diffusion of organic vapors in high polymer substances, the diffusion coefficient depends on the concentration of diffusing substance. In this program, we assumed that the diffusion coefficients are independent of the concentration of diffusing substance.
2. Diffusion coefficient may vary with location if the porous medium is not homogeneous. The porous medium in this program is considered homogeneous.
3. This model assumes the diffusion of the chemical in the soil is strictly one-dimensional.
Bulk Density: Bulk density is the ratio of the mass of dried soil to its total bulk volume (solids and pores together).
Diffusion coefficient: Diffusion coefficient is a parameter expressing the transfer rate of a substance by random molecular motion. Mathematically, it is defined as the specific transfer rate under a unit driving concentration gradient.
Diffusing substance: A diffusing substance is a component species in a diffusing system. For example, in a table salt solution, both sodium and chloride ions are diffusing substances.
Effective diffusion coefficient: Effective diffusion coefficient is the diffusion coefficient in an adsorbing system. It equals the diffusion coefficient of a chemical obtained under non-adsorbing conditions divided by a retardation factor of the adsorbing system.
Mixing depth: Mixing depth is a depth in a soil profile to which a diffusing substance is initially distributed.
Molecular diffusion: The net transfer of mass by random molecular motion. This net mass transfer is caused by gradients in concentrations of a diffusing substance.
Organic carbon content: Organic carbon content of a soil is the amount of organic carbon in unit dry mass of the soil. It is usually expressed as a percentage of the soil dry mass.
Organic carbon partition coefficient: Organic carbon partition coefficient is the partition coefficient in a soil divided by the organic carbon content of the soil.
Partition coefficient: Partition coefficient is the ratio of the concentration of a chemical species adsorbed on a soil to the concentration of the species in the soil solution.
Retardation factor: Retardation factor is a dimensionless parameter characterizing the retarding effect of adsorption on solute transport. Mathematically, the retardation factor, R, is defined as R=1+rKd/q, where r is bulk density, Kd is partition coefficient, and q is volumetric water content.
Specific transfer rate: Specific transfer rate is the transfer rate per unit cross-sectional area. That is, it is the flux density
Volumetric Water Content: The volume of water in a soil divided by the total volume of the soil (i.e. the sum of the volumes of solids and pores).
Porosity: The volume of soil pores divided by the total volume of the soil (i.e. the sum of the volumes of solids and pores).
Allen III, M. B., I. Herrera, G. F. Pinder, 1988. Numerical modeling in science and engineering. John Wiley & Sons, Inc., New York.
Carslaw, H. S. and J. C. Jaeger, 1967. Conduction of heat in solids. Oxford University Press, New York, p. 54-56.
Crank, J., 1956. The mathematics of diffusion. Oxford University Press, New York, p. 12-15.
Kirkham, D. and W. L. Powers, 1972. Advanced soil physics. Wiley-Interscience, A division of John Wiley & Sons, Inc., New York.
Millington, R. J. and J. M. Quirk, 1961. Permeability of porous solids. Trans. Faraday Soc. 57:1200-1207.
Contributors
This program was designed by Dr. D. L. Nofziger and Dr. J. Wu, Department of Plant and Soil Sciences, Oklahoma State University, Stillwater, OK 74078.
Send email to david.nofziger@okstate.edu
Last Modified: January 16, 2008.