degradation
Degradation: Chemicals applied to soils may undergo biological or chemical transformations, be taken up by plants, escape to the atmosphere in vapour form, be lost to surface water by surface water flow and erosion, or remain in the soil and be available for leaching to groundwater. The importance of these different processes depends upon the chemical of interest.
Active ingredients in most pesticides break down or degrade over time as a result of microbiological and chemical reactions. The persistence of a pesticide refers to its "staying power" in the soil profile. Pesticides with low persistence degrade quickly to other products. Most biological breakdown occurs in the root zone of the crop since more microbes exist there. Pesticide degradation rates also depend upon soil temperature and water content.
Pesticide persistence is usually specified in terms of the degradation half-life. Half-life is a measure of the time it takes for one-half of the original amount of pesticide in a soil to be deactivated. Degradation of this type is called "first order degradation" and can be represented mathematically as
![]()
where m(t) represents the amount of pesticide at some time t and m(to) represents the amount at an earlier time, to. If the half-life of the chemical is known, we can calculate the amount of pesticide remaining in the soil at any time of interest using this equation with to = 0 and m(to) equal to the amount of pesticide applied.
The following figure illustrates the shape of these first-order degradation curves for half-life values of 14, 20, and 90 days. These half-life values are typical values for acifluorfen sodium salt, bentazon sodium salt, and metolachlor, respectively. The curves show the amount of pesticide remaining at different times if 1 kg of each product was applied to the soil.
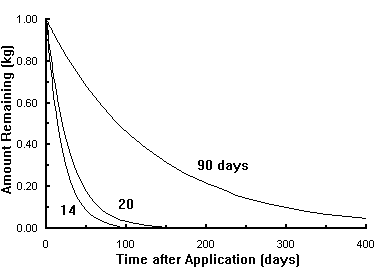
If first-order degradation describes pesticide degradation, 3.3 half-lifes must pass before the amount of pesticide remaining is reduced to 10% of its original amount, 6.6 half-lifes to reduce it to 1%, and 10 half-lifes to reduce it to 0.1% of the original amount. The amount of degradation needed depends upon the initial amount applied, the toxicity of the product, and the manner in which it mixes in the aquifer. An program is available here to enable you to observe the predicted degradation process if first-order degradation is followed and the degradation rate is constant with time and depth.
Half-life values are usually determined for surface soils. Degradation rates are generally smaller and half-life values are larger in subsoils where less microbial activity occurs. Therefore, as pesticides are leached to lower depths, they degrade more slowly. This can be significant if a large storm occurs or the area is irrigated soon after a pesticide is applied since such events may leach the chemical before the surface organisms degrade it.
The degradation rate varies with temperature. In general, the rate decreases with decreasing temperature for temperatures in the range encountered in most soils. Interactive software is available to enable you to observe the manner in which soil temperatures change with time of year and with depth and the impact of changes in temperatures upon degradation.